June 25, 2013, by Graham Kendall
What is Pharmaceutical Nanoemulsion?
In this What is? series, we invite experienced researchers to write about their area of research, but in more general terms than they might normally do.
This article, entitled What is Pharmaceutical Nanoemulsion?, is written by Prof. Sivakumar Manickam
If you would like to contribute, then please let me know (Graham.Kendall@nottingham.edu.my) and we can discuss how best to go about it.
Anybody wishing to contact the author of this article should go to about the author.
What is Pharmaceutical Nanoemulsion?
Design and Manufacture of Pharmaceutical Nanoemulsions using a Green Processing Technique – Ultrasound Cavitation
One of the nanoscience approaches that has receiving an increasingly considerable attention within the pharmaceutical sciences is the formulation as nanoemulsions, mostly oil-in-water (O/W) type as it appears to be an alternative yet effective dosage forms for poorly water-soluble drugs, in which these active pharmaceutical ingredients may be dissolved into inner phase of the emulsion. Nanoemulsion formulations are extensively studied for their potential application as multifunctional nanocarriers which allow the treatment of a variety of pains and diseases. However, the preparation of such fine emulsions with small droplet size can be a challenge since the emulsion properties such as stability, color, appearance, texture, and rheology are greatly affected by emulsion droplet size. Over the recent years, interest has grown in the use of ultrasound cavitation in the design and development of new dosage form of pharmaceutical nanoemulsion formulations. Much has been reported about the advantages of making lipophilic active ingredient into nanoemulsion dosage form as potential drug delivery system. The common consensus is that they are better and even superior than macroemulsions because of their versatile features and unique advantages. They often characterized by their increased drug solubility, rapid dissolution velocity, and enabling bioavailability after oral administration due to their much larger interfacial area to volume ratio. The aim of this blog is to strive to give an overview about ultrasound cavitation and the underlying the formation of pharmaceutical nanoemulsion.
What is Pharmaceutical Nanoemulsion (NE)?
Pharmaceutical emulsion is usually a homogenous mixture consisting of various oils and/or fats intimately dispersed throughout the aqueous continuous phase in the presence of an emulsifying agent (also called emulsifier or emulgents) that are “Generally Recognized as Safe” (GRAS) by FDA . The type of emulsion formed, normally oil-in-water (o/w) or water-in-oil (w/o), is commonly determined by the volume ratio of the two liquids and also by the phase addition sequence and by the nature of emulsifier. The former type finds a wide area of applications in oral and topical formulations of oil-soluble drugs as it is not only pleasant to take/use but also provides a remarkable masking effect with the inclusion of a suitable flavour. Whereas, the latter type is only useful for the preparation of cosmetic products like moisturizing creams. In this case, it produces a layer of coherent and water-proof film that efficiently prevents moisture loss from the skin. Recently, nanotechnology-based drug delivery systems such as nanoemulsions have emerged to increase the dissolution rates and bioavailability of a myriad of drugs that are poorly soluble in water with reduced possible side effects.
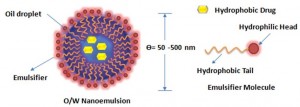
By definition, nanoemulsions are isotropic and kinetically stable emulsion systems in which the oil droplets containing the hydrophobic drug are stabilized by a thin layer of emulsifier. They appear to be either transparent (droplet diameter <200nm) or milky (droplet diameter ≈ 500nm) with the mean droplet diameters ranging from 50 to 1000 nm. The average droplet sizes of nanoemulsion, say oil-in-water type, fall typically in the range of 100-500 nm. They are also referred to as mini-emulsions, ultrafine emulsions or submicron-emulsions. Their long term physical stability associated with small nanosized droplet that enables the system to remain dispersed with no apparent flocculation or coalescence and therefore they are sometimes referred to as ‘approaching thermodynamic stability’. Thermodynamically speaking, nanoemulsion formation is a non-spontaneous process and a large amount of emulsifier or energy is required to break a droplet into smaller ones (submicron, as is the case with nanoemulsions). Their preparation is, therefore, restricted to the use of high energy emulsification devices such as ultrasonic generators, high pressure homogenizer or microfluidizer. The high energy needed for such fine emulsions can be expressed in terms of Laplace pressure, p (the difference in pressure between inside and outside droplet).
Formulation Aspects of Nanoemulsion
Generally pharmaceutical nanoemulsions consist of oils, emulsifiers and co-emulsifiers as excipients in their preparation or formulation strategy. It should be noted that the combination of these three components are essential to impart and control physical stability and consistency of nanoemulsion in the formulations. The oil phase of nanoemulsion plays a vital role in nanoemulsion formulation as it can solubilize highly potent lipophilic drugs to facilitate their transport via the intestinal lymphatic system. PUFA-rich edible plant-seed oil, such as flax-seed oil, safflower oil, castor oil, soybean oil, and maize are rich in Omega-3 and Omega-6-fatty acids and contain useful vitamins and minerals. They are readily ingested and virtually do not present safety issues. They are often, therefore, formulated for oral administration. In many instances, it is to be noted that nonionic emulsifiers are used in nanoemulsion formulations because of their tendency to be less toxic and irritant than their anionic, and particularly their cationic, counterparts. They are usually characterized by their good solvent capacity and thus accepted for oral ingestion. In most of the cases, the addition of coemulsifier, an amphiphile with an affinity for both the oil and aqueous phase is necessary for the formation of nanoemulsion as the single chain emulsifiers are generally not able to reduce the o/w interfacial tension solely and sufficiently to an appreciable extent. The penetration of coemulsifier into the emulsifier interfacial film at the o/w interface helps to further reducing the fluidity of the interface and thereby increasing the entropy of the entire colloidal system.
The Ultrasonic Domain
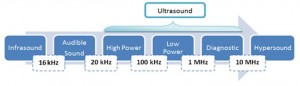
Ultrasound spans the frequencies of roughly 15 kHz to 1 GHz with typical sound velocities in liquids of about 1500 m/s and acoustic wavelengths range roughly from 10 to 10-4 cm. Within the ultrasound range, the power available varies inversely with the frequency and only powerful ultrasound (16 – 100 kHz) is able to produce physical and chemical changes such as emulsification. Today ultrasound is widely used and applied in industries for plastic and metal welding, machining, soldering and sonocleaning and even in hospitals for diagnostic and medical imaging. Intriguingly, in the pharmaceuticals and medicine, the unique advantages of ultrasonic drug delivery have recently been explored and employed in several tissue systems, including dermal and transdermal delivery, localized delivery to tumours, and in some cases, it has been used to increase the rate of drug transport and release at the targeted site.
What is ultrasound cavitation?
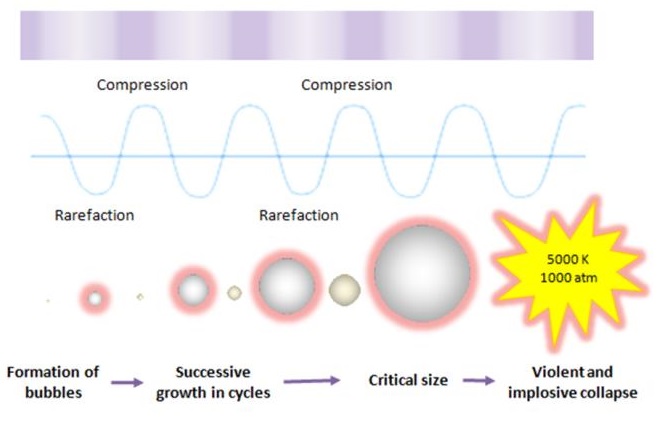
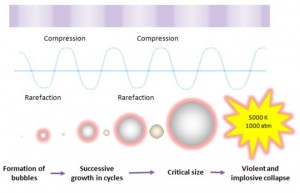
Ultrasound cavitation may be defined as the formation, growth, and implosive collapse of bubbles in a liquid medium. Inception of cavitation bubbles generally originate from nuclei, especially those micro-bubbles present in the bulk of the liquid. The compression of gases and vapors inside the cavity initiates inertial bubble collapse phase and generates intense heat and thereby creates a short-lived, localized hot spots which have an equivalent temperature of approximately 5000 K, pressures of about 1000 bar, and heating and cooling rates above 1010 K/s. The enormous amount of energy arising from the extreme physical and chemical conditions of these hot-spots are deemed to serve as the impetus of a vast majority of sonochemical reactions and this high energy concentrating effect is responsible to accelerate and enhance the chemical and biological processes.
This cavitation event gives rise to an extreme level of highly localized turbulence driven by the millions of intense shock waves generated at the implosion sites. The collapse of these micro-implosions induce powerful shock waves to radiate throughout the solution in proximity to the radiating face of the horn tip, thereby breaking up the larger primary oil droplets into a liquid medium. It is clear that the violent and implosive collapse provides a couple of strong physical effects outside the bubble: shear forces, liquid jets and shock waves which effectively dispersing the oil droplets into a continuous liquid phase.
Ultrasound Emulsification
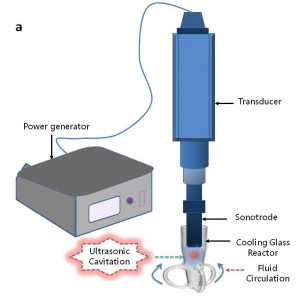
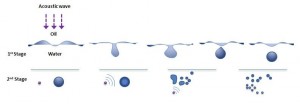
Ultrasound emulsification occurs when one liquid dispersed into another immiscible liquid under the influence of an acoustic field. The acoustically-formed emulsions have been surprisingly been discovered to possess an unusual stability even without the presence of an emulsifier. Furthermore, the mean droplet size of resulting emulsions is extremely small and generally falls into the submicron region. In an ultrasonic field, the intense shock waves produced in the surrounding liquid caused by imploding cavitation bubbles and the formation of liquid jets at high velocity were responsible for the formation of emulsion droplets. In normal circumstances, a two-stage ultrasound emulsification mechanism of liquid-liquid system can be used to explain the influence of ultrasound energy on droplet formation and disruption
- The first stage primarily involves a synergistic action of interfacial waves of acoustic field and Rayleigh-Taylor instability, which eventually lead to the eruption of the oil phase into water medium to form the initial larger droplets.
- The second stage is based on the deformation and subsequent break-up of these larger droplets into submicron range due to the impact of cavitation-induced shock waves generated near the interface when subjected to an acoustic field.
In the emulsification process, ultrasound had emerged as an excellent yet powerful emulsifying tool as compared to that of other mechanical alternatives in terms of obtaining a smaller droplet size. Additionally, under the same conditions, in the case of producing a nanoemulsion with desired diameter, the required amount of surfactant was significantly reduced; the energy consumption was considerably lower than other classical mechanical devices. It would, then, appear that ultrasound emulsification may be used in place of high-pressure homogenization and microfluidization as it is capable of achieving similar local power densities with lower operating costs, if a suitable treatment chamber is used.
Success stories in our Laboratory
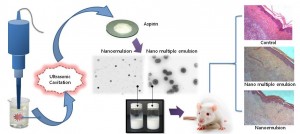
In our laboratory, we have generated a variety of nanoemulsions encapsulated with different pharmaceuticals such as Aspirin, Curcumin and Ganoderic acid.
Furthermore, the anti-inflammatory activities of nanoemulsion were determined using the λ-carrageenan-induced paw edema model. The analgesic activities of both nanoformulations were determined using acetic acid-induced writhing response and hot plate assay. For comparison, the effect of pretreatment with blank nanoemulsion and reference Aspirin suspension were also studied for their anti-inflammatory and antinociceptive activities. The results showed that oral administration of nanoemulsion containing Aspirin (60 mg/kg) significantly reduced paw edema induced by λ-carrageenan injection. Nanoformulations decreased the number of abdominal constriction in acetic acid-induced writhing model. Pretreatment with nanoformulations led to a significant increase in reaction time in hot plate assay. Nanoemulsion demonstrated an enhanced anti-inflammatory and analgesic effects compared to reference suspension. These experimental studies suggest that nanoemulsion produced a pronounced anti-inflammatory and analgesic effect in rats and may be candidates as new nanocarriers for pharmacological NSAIDs in the treatment of inflammatory disorders and alleviating pains.
Studies to date suggesting that in the emulsification process, ultrasound assisted cavitation appears to be more competitive or even superior in terms of droplet size and energy efficiency compared to other typical rotational homogenizers. Ultrasound appears to be more economical and practical in terms of scale-up production cost, ease of maintenance, and aseptic processing. In general, in the emulsification process, ultrasound had emerged as an excellent yet superior tool as compared to rotor stator in terms of obtaining a smaller droplet size and high energy efficiency. Additionally, the results indicated that, in the case of producing an emulsion with desired diameter, the required amount of surfactant was significantly reduced; the energy consumption was considerably lower than other classical mechanical devices. More interestingly, the acoustically formed emulsions were all found to be stable excellently and more homogeneous as compared to mechanical methods. Furthermore, mean droplet size and polydispersity index of the aspirin nanoemulsions can be produced in a controlled manner by varying the formulation parameters and the ultrasonic processing variables. Most importantly, the present study confirmed that the ultrasonic cavitation is relatively a simple, effective and powerful emulsification technique for producing aspirin nanoemulsions with minimum mean droplet diameter of about 200 nm.
About the author
Sivakumar Manickam is a Professor of Chemical and Nanopharmaceutical Process Engineering and his areas of expertise are, Nanoengineering of Advanced Materials (Synthesis, characterization and process development); Development of Nanosuspensions and Nanoemulsions for Pharmaceutical Industries; Utilization of highly Energy Efficient Cavitation Technique for the Synthesis of Nanomaterials and wastewater treatment. He has about 20 years of experience in the pharmaceutical process engineering and cavitation technologies and is consultant to many process industries. He has published about 150 journal papers and conference presentations. He is heading the Manufacturing and Industrial Processes Research Division and Coordinator of the Centre for Nanotechnology and Advanced Materials (CENTAM).
Contact details
- URL: http://www.nottingham.edu.my/Engineering/Departments/Chemenv/People/sivakumar.manickam
- http://www.nottingham.edu.my/Engineering/Research/Manufacturing/Index.aspx
- EMAIL: Sivakumar.Manickam@nottingham.edu.my
References
- S. Y. Tang, K. H. Tan and M. Sivakumar (2011) Ultrasound Cavitation as a Green Processing Technique in the Design and Manufacture of Pharmaceutical Nanoemulsions in Drug Delivery System. In Green Chemistry and Environmental Remediation, Chapter 7, 179-180, Hoboken, NJ: Wiley-Scrivener.
- S. Y. Tang and M. Sivakumar A Novel Formulation and Optimization of Aspirin Nano-emulsion prepared by Cavitation induced by Ultrasonic waves. Journal of Industrial Technology, 19(1), 179-196, 2010. Publisher: SIRIM.
- S. Y. Tang, M. Sivakumar, K. H. Tan, and B. Nashiru Formulation Development and Optimization of a Novel Cremophore EL-based Nanoemulsion using Ultrasound Cavitation. Ultrasonics Sonochemistry, 19(2), 330-345, 2011.
- S. Y. Tang and M. Sivakumar Design and Evaluation of Aspirin-loaded Water-in-oil-in-water Nano Multiple Emulsions Generated using Two-stage Ultrasonic Cavitational Emulsification Technique. Asia-Pacific Journal of Chemical Engineering 2010 Special Issue. 7(S1), S145-S156, 2012.
- S. Y. Tang, M. Sivakumar, M. H. Ng, and P. Shridharan (2012) Anti-inflammatory and Analgesic Activity of Aspirin of Novel Oral Nanoemulsion and Nano Multiple Emulsion Formulations Generated using Ultrasound Cavitation Technique. International Journal of Pharmaceutics, 430, 299-306.
- S. Y. Tang and M. Sivakumar (2013) A Novel and Facile Liquid Whistle Hydrodynamic Cavitation Reactor (LWHCR) to Produce Submicron Multiple Emulsions. AIChE Journal, 59(1), 155-167.
- S. Y. Tang, M. Sivakumar, and P. Shridharan (2013) Effect of Process Parameters in the Generation of Aspirin Nanoemulsions – Comparative Studies between Ultrasound Cavitation and Microfluidizer. Ultrasonics Sonochemistry, 20(1), 485-497.
- S. Y. Tang, M. Sivakumar and B. Nashiru (2012) Impact of Gelling, Emulsifiers and Osmotic Pressure in the Generation of Highly Stable Single Core Water-in-Oil-in-Water (W/O/W) Nano Multiple Emulsions of Aspirin assisted by Two-stage Ultrasonic Cavitational Emulsification. Colloid and Surfaces B: Biointerfaces, 102, 653-658.
- Tan Khang Wei and Sivakumar Manickam (2009), “Synthesis and Characterization of Ganoderic Acid Nanoemulsion or Reverse Nanoemulsion as Colloidal Carrier”. International Conference on Nanotechnology Research and Commercialization (ICONT), 14th-17th December, Langkawi, Malaysia.
- Tan Khang Wei and Sivakumar Manickam (2010) “Fabrication and Characterization of Curcumin Nanoemulsion as Colloidal Carrier”. International Conference and Exhibition on Pharmaceutical, Nutraceutical and Cosmeceutical Technology (Pharmatech), 25th-26th May, Kuala Lumpur, Malaysia.
- Tan Khang Wei and Sivakumar Manickam (2010) “Optimization for the Preparation of Curcumin loaded Nanoemulsion using Response Surface Methodology”. 13th Asia Pacific Conference of Chemical Engineering Congress (APPChE), 5th-8th Oct, Taipei, Taiwan.
No comments yet, fill out a comment to be the first


Leave a Reply