December 16, 2020, by Guest Blogger
The pharmacist’s role in the Mass COVID Vaccination Programme in Nottingham
This guest blog from Jessica a pharmacist and student of UoN gives us an insight in to the other side of the vaccination process in Nottingham, and what it’s like to train for, and deliver them.
Just prior to the national announcement of the arrival of Pfizer’s COVID-19 mRNA Vaccine BNT162b2 I was invited to register my interest as a pharmacist to help work at one of the vaccination sites across Nottingham. Due to my current role in the NHS, as an independent prescriber in general practice, they were able to fast track my application and necessary checks and indemnity. I was so excited by the arrival of the vaccine I signed up for a weekend shift straight away which I could fit around my PhD studies and general practice commitments.
The day before my first 12 hour shift, which I haven’t done in a long time, I was asked to attend the vaccination centre at the King’s Mill hospital, part of the Sherwood Forest Hospitals NHS Foundation Trust, for a run through of the protocols and procedures. At that point we were not sure how many pharmacists were available at the weekend but they assured me that there would be a resident pharmacist available throughout the day with access to the vaccine and associated supplies which were stored in the hospital’s pharmacy. In preparation for the shift I had to complete a number of online training modules specific to the COVID-19 vaccination. I have been vaccine trained for 5 years now and make sure that my immunisation, anaphylaxis and basic life support training is updated on an annual basis.
There was one clinic room in operation on the day and a pharmacist had to be present at all times in order for the vaccinations to be administered. The Pfizer COVID vaccine has been approved safe for use but has not yet been granted a full license. The MHRA has been able to enact Regulation 174 which in exceptional situations enables the temporary authorisation of the supply of an unlicensed medical product in response to certain identified public health risks, such as the SARS-CoV-2 pandemic.
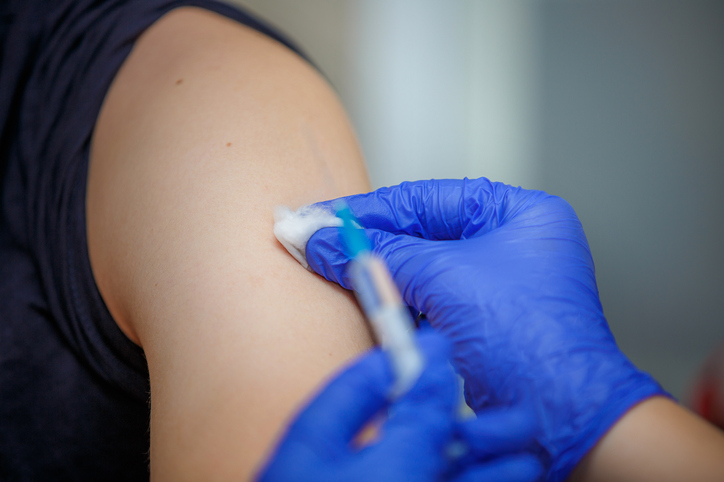
Our main role was to ensure that the vaccine had been transferred from the ultra-low temperature freezer to the fridge 3 hours prior to use. Each vial had to be checked and accounted for and taken out of the fridge 10 minutes prior to reconstitution and keep out of direct light. We then had to do quantity and batch number checks throughout the reconstitution procedure, checking the quantity and quality of each individual syringe prepared. The correct timings needed to be recorded as the reconstituted vial must be used up within 6 hours. Needless to say we got through each vial in a matter of minutes, never mind hours, as the flow of patients kept coming.
The patients and staff were in good spirits, some patients even asking to have their picture taken whilst they were receiving the vaccine. For some, this was a keepsake of their experience in this significant moment in history and for others it was a way of encouraging their loved ones and carers to also get vaccinated. The excitement and gratitude was palpable and we experienced no serious side effects on the day after delivering nearly 250 doses. We actually ended up with an abundance of pharmacists compared to nurses so I was able to use my vaccination training to help deliver the vaccines alongside the nursing team. Meanwhile, the healthcare assistants, administration staff, security staff, medical and non-medical prescribers worked in the room next door completing the paperwork, signing the patient-specific directions (PSDs) and organising the flow of patients. It really was multi-disciplinary working at its best and a satisfying and buoyant day for all those involved and I am looking forward to another shift next weekend!
For more on UoNs response to the Coronavirus pandemic, please visit our Covid-19 webpages here
No comments yet, fill out a comment to be the first

Leave a Reply