
August 3, 2021, by Emma Lowry
Researchers are first in the world to watch plants ‘drink’ water in real-time
Scientists at the University of Nottingham have become the first in the world to find a way to observe how plant roots take in and circulate water at the cellular level, which could help to identify future drought- and flood-resistant crops.
The inability to monitor water uptake inside roots – without damaging the specimen – has been a key stumbling block for researchers seeking to understand the motion of fluids in living plant cells and tissues.
Study lead, Dr Kevin Webb from the Optics and Photonics Research Group, explains, “To observe water uptake in living plants without damaging them, we have applied a sensitive, laser-based, optical microscopy technique to see water movement inside living roots non-invasively, which has never been done before.
“Fundamentally, the process by which plants are able to thrive and become productive crops is based on how well it can take up water and how well it can manage that process. Water plays an essential role as a solvent for nutrients, minerals and other biomolecules in plant tissues. We’ve developed a way to allow ourselves to watch that process at the level of single cells. We can not only see the water going up inside the root, but also where and how it travels around.
“Feeding the world’s growing population is already a problem. Climate change is causing huge shifts in the pattern and density of waterfall on the planet which leads to problems growing crops in regions hit by floods or droughts. By selecting plants that are better at coping with stress, the goal is to increase global food productivity by understanding and using plant varieties with the best chances of survival that can be most productive in any given environment, no matter how dry or wet.”
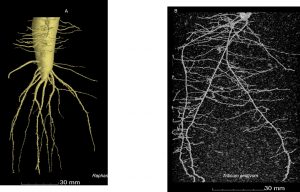
For the study, water transport measurements were performed on the roots of Arabidopsis thaliana, which is a ‘model plant’ for scientists since they can be easily genetically-engineered to interfere with basic processes like water uptake.
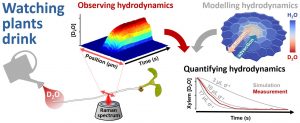
Using a gentle laser, the new imaging technique – based on the Nobel Prize-winning Raman scattering technique – allowed researchers to measure water travelling up through the root system of Arabidopsis at the cellular level, and to run a mathematical model to explain and quantify this.
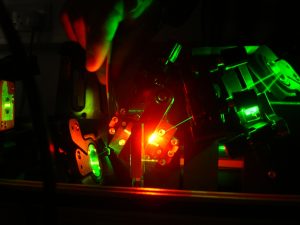
Conversion of high intensity green light into bio-friendly red wavelengths, within the Titanium:Sapphire laser
The researchers used ‘heavy’ water (deuterium oxide, or D2O), which contains an extra neutron in the nucleus of each hydrogen atom. By scanning a laser in a line across the root while the plant drank, it was possible to see the ‘heavy’ water moving past via the root tip.
In Arabidopsis that had been genetically-altered to compromise its water uptake, these measurements – combined with the mathematical model – revealed an important water barrier within the root. This confirmed for the first time that water uptake is restricted within the central tissues of the root, inside of which the water vessels are located.
Co-lead, Malcolm Bennett, Professor of Plant Sciences at the University, said, “This innovative technique is a real game-changer in plant science – enabling researchers to visualise water movement at a cell and second scale within living plant tissues for the very first time. This promises to help us address important questions such as – how do plants ‘sense’ water availability? Answers to this question are vital for designing future crops better adapted to the challenges we face with climate change and altered weather patterns.”
The findings of this Leverhulme Trust-funded study, are published in the journal Nature Communications in a paper entitled: ‘Non-invasive hydrodynamic imaging in plant roots at cellular resolution’.
Future applications
While developing the method, the research initially focused on plant cells, which are about 10 times the size of human cells and therefore more easily observed. The research team is currently porting these same methods to human cells to understand exactly the same kinds of processes at an even smaller scale.
Just as with plants, there are tissues in the human body responsible for handling water, which is crucial to function. Transparent tissues of the eye, for example, can suffer from diseases of fluid handling which include ocular lens cataracts; macular degeneration and glaucoma. In future, the new Raman imaging technique could become a valuable healthcare monitoring and detection tool.
Next steps
The researchers are working towards a commercial path for their hydrodynamic Raman imaging technique, and have just applied for funding with four UK and EU agriculture companies to look at tracers that move from plant leaves to roots to understand both directions of water transport. In parallel, the team is working on portable versions of the technology to allow water transport measurements to be taken into the field by farmers and scientists to monitor water handling in crops growing in challenging local environments.
The research team is currently bidding for a European Research Council Synergy Grant with partners in the EU and UK to take the study of water uptake and drought resistance towards being a new tool to help choose and understand how particular crops can be matched to particular local growth conditions.
ENDS
More information and / or a copy of the paper are available from Dr Kevin Webb on kevin.webb@nottingham.ac.uk (optics/physics) or Professor Malcolm Bennett on malcolm.bennett@nottingham.ac.uk (plant biology) or Emma Lowry, Media Relations Manager (Engineering) on +44 (0) 115 8467156 or emma.lowry@nottingham.ac.uk
Notes to editors
Eight laboratories across Europe were involved in the study: the Optics & Photonics Research Group; the Future Food Beacon and the Division of Plant and Crop Sciences at the University of Nottingham; the Centre for Plant Integrative Biology; School of Biological Sciences, University of Bristol; Institut Agro, France; Earth and Life Institute, Belgium; Excellence Unit Agrienvironment, University of Salamanca, Spain; Departamento de Anatomía, Biología Celular y Zoología, Universidad de Extremadura, Spain.
No comments yet, fill out a comment to be the first

Leave a Reply