
May 4, 2018, by Melissa Wadams
Complexity and logistics of enrolling children onto clinical trials – a reflection on BBC2’s #Hospital Episode 2
In this blog, Richard Grundy, Professor of Paediatric Neuro-Oncology at the Children’s Brain Tumour Research Centre, shares his insights into the importance and complexities of clinical trials involving children with brain tumours.
Having recently taken part in the filming for the BBC2 programme, Hospital, which featured a patient, who was accepted onto the SIOP PNET5 MB trial1 during filming, I am able to reflect on the complexity of enrolling children with brain tumours onto clinical trials, and the limitations of this current system for researchers.

© BBC Hospital
We have unique opportunities in the UK: near-universal treatment within a single healthcare system; internationally leading national cancer registration systems; an established CRUK funded national tissue bank through CCLG centres; a trials-driven professional workforce, a networked imaging database; and close, multidisciplinary integration at a national level between clinical trials, cancer intelligence and cancer service commissioning and delivery. Despite these advantages, childhood brain tumour survival, particularly for high-grade tumours, lags behind apparently similar European and US neighbours.
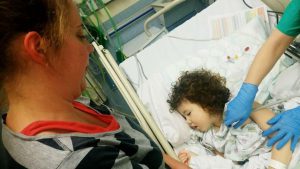
Rona McGill at Poppy’s bedside ©NottinghamPost
The child featured in the programme, Poppy, is one of the first 3 patients to have been enrolled onto the PNET5 trial in the UK. For Poppy to be able to be enrolled into the trial, it involved a huge multi-disciplinary effort from the staff in Nottingham Children’s Hospital. This included molecular profiling, (the biology of her tumour determines which trial arm she can access), surgical teams ensuring that adequate tissue during surgery is collected and frozen immediately, and detailed paperwork collected by the nursing team. Poppy drew the carboplatin arm. In this trial it is being used as a radiosensitiser to improve the effectiveness of radiotherapy. This has to be given between 1 and 3 hours before the chemotherapy, so for Poppy, who needed a general anaesthetic that has to be given at 08.30 every morning, this posed a logistical nightmare. The regional children’s cancer services worked incredibly well to make these nightmarish logistics happen for this family. A huge credit to the dedication and teamwork of our team at during an incredibly busy time within Nottingham Children’s Hospital and Nottingham University Hospitals NHS Trust.
During an incredibly busy time within Nottingham Children’s Hospital, the multi-disciplinary team involved was able to achieve a great opportunity for Poppy and her family. But, this is not the case for every patient. Presently, there is significant variation in neurosurgeons’ attitudes and practice of tumour storage for research in the UK14. Indeed only 50% of CNS tumours have tumour tissue frozen at diagnosis, which potentially prevents patient entry into a number of clinical trials which mandate this. However, things are improving. In the Ependymoma trial, which I am running, we have at least 80% of cases with fresh frozen tissue. The clinical trial is very carefully monitored and all new cases are discussed in our national Ependymoma Multi-disciplinary Advisory Group. This close scrutiny of cases has resulted in a significant improvement in the surgical resection rate of these tumours from 50% to 85%. As a complete surgical resection is one of the strongest predictive markers for a good outcome, we would hope that this will translate into better outcomes for Children with this, presently, very difficult to cure tumour. This highlights the value of national advisory groups in improving outcomes for childhood brain tumours.
To find out how Poppy’s treatment is progressing and her recovery, see her family’s website
You can watch clips from the episode on iplayer here
By Richard Grundy, Professor Paediatric Neuro-Oncology, Children’s Brain Tumour Research Centre

Picture: Alex Cantrill-Jones / ACJ Media
No comments yet, fill out a comment to be the first

Leave a Reply