November 27, 2020, by School of Medicine
50 at 50: Reflections on directing the NIHR Health Technology Assessment Programme and being a Welsh sheepdog during the pandemic
On September 30th 2020, I stepped down as the Director of the NIHR HTA Programme after five very busy years. To set the scene, The NIHR Health Technology Assessment (HTA) Programme sits within the National Institute for Health Research (NIHR) – the UK’s largest funder of health and social care research funded by the UK Government. HTA is the largest NIHR Programme and in 2018/19, it supported 584 live projects worth £0.5 billion – that’s 268 active clinical trials, 74 new projects, 86 final reports, 40,567 participants recruited, 1,635 peer reviewers, 1,915 topic suggestions received and 67 calls advertised with an annual spend £76 million. Quite a responsibility then to direct HTS such a programme, so what are my reflections?
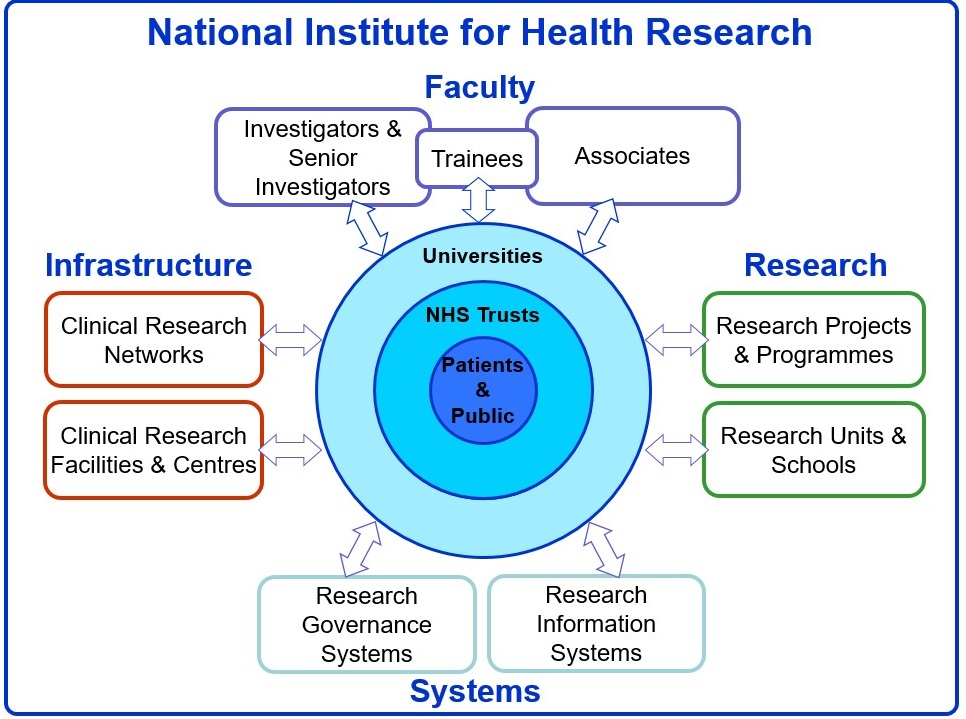
The first is that I remain convinced that our National Institute of Health Research is by far the best research system in the world. Seriously, no other global research system has all of these key components joined up: a faculty of skilled researchers, funding programmes, research governance systems and an amazing infrastructure to deliver research – and with NHS patients clearly at the centre (Figure 1). Clinical research is now everyone’s business in the NHS. A unique aspect of the HTA Programme has been to work with patients and the public and health care professionals to identify questions that our NHS needs answering, rather than just reacting to researcher-led proposals, although the two streams work together in a creative tension to close any gaps.
The second is to tell you what a fantastic bunch of people I have had the privilege to work with. The support team at NETSCC, our leaders at the DHSC, and all who served on our funding committees have their hearts in the right place. Egos are left at the door in our funding committees and there is a real shared vision of wishing to make things better for our NHS patients.
Third, such a responsible role has given me an opportunity to change things – hopefully for the better. Some of the things that I have taken a particular interest in promoting through teamwork include:
- Ensuring strong and genuine patient and public involvement in all our committees
- Redressing inequalities in research –like encouraging recruitment where the burden of disease is greatest and increasing diversity in our funding committees
- Reducing research waste by ensuring all our topics are prioritised, well designed and that all are reported fully in a timely fashion in the public domain, and by introducing the concept of enhanced dissemination
- Celebrating our commissioned work stream –tackling market failures as well as new technologies so that they can be used by our NHS
- Encouraging use of up to date methodology such as data-enabled trials or studies within a trial and core outcomes to be used where they exist
- Promoting more cross-programme working so that good ideas are not lost by falling between the programmes
 I have tried to be as fair as I can to all research communities with no hidden agendas and my communications have been through monthly YouTube videos and are accessible to all. I can honestly say that I have played with a straight bat from start to finish and it has been a privilege to serve the British public in this way.
I have tried to be as fair as I can to all research communities with no hidden agendas and my communications have been through monthly YouTube videos and are accessible to all. I can honestly say that I have played with a straight bat from start to finish and it has been a privilege to serve the British public in this way.
Since the start of the pandemic in early 2020, I have also been involved in several COVID-19 research initiatives. The first was to protect the HTA Programme, ensuring that those trials that were struggling to recruit just before the lockdown were given opportunities to recover. Some major changes to study design have been needed eg delivering a talking therapy intervention online rather than face to face. Such a change can affect intervention intensity, fidelity and adherence and hence study power, so we have taken a pragmatic and structured approach into making such adaptions when possible. Major protocol changes in the light of such extenuating circumstances need to be reported carefully, and to this end I am involved in an extension of the CONSORT and SPIRIT reporting guidelines group that called CONSERVE. They will make recommendations of what key items to report and how and will be reporting soon.
I also joined the Urgent Public Health Oversight Group for COVID-19 research – a group that tries to join and streamline the many initiatives around prioritising COVID-19 research. I then took part in the UKRI/NIHR rapid research panel that funded some good studies. One of the most interesting groups I joined is called RAPID-19 – a collaboration between NICE, MHRA, NIHR and NHS England and the devolved nations to speed up the evaluation and approval pathways for effective medicines for NHS benefit. It has worked really well so far due to early readouts and good collaboration – resulting in NHS patients being given dexamethasone and remdesivir within 24 hours of rapid approvals. Given my role in so many key COVID019 oversight groups, the DHSC have asked me to stay on to ensure continuity and connectivity for the next 12 months. I am now also involved in a Prophylaxis oversight group, exploring the case for national Prophylaxis studies, and I am also part of the task force for future NIHR emergency planning.
Looking back over the last 6 months, it has been quite mad and exhausting at times. Annual leave has been a joke, and I have worked every Saturday and Sunday to date – as do most of my NIHR oversight colleagues. With so many new initiatives springing up, I have felt at times like a Welsh sheepdog running between committees to keep everyone informed and working together. There have been some downsides – too much emphasis on drug treatments and not enough research on mental health consequences of the pandemic or rehabilitation. There has perhaps been too much COVID-19 research at the expense of non-COVID-19 research, and a real risk of overlap and non-completion of projects in the face of limited CRN capacity which will need to contend with a collision of therapeutic trials, vaccine trials, prophylaxis trials and the whole range of non-COVID research as we enter the second wave. I also feel that there has been too much glory-seeking at times from single institutions, rather than working across the country in a more collegiate fashion. But these concerns are minor compared to the tremendous dedication and great teamwork I have observed, delivering needed research in a timely way for the benefit of the British people and beyond. A lot of very good work has been done a short time and we have learnt many lessons along the way.
By Professor Hywel Williams, Professor of Dermato-Epidemiology & Co-Director of the Centre of Evidence Based Dermatology
No comments yet, fill out a comment to be the first

Leave a Reply