« return to post
Journal-sq2
(b) GMP derivatives with
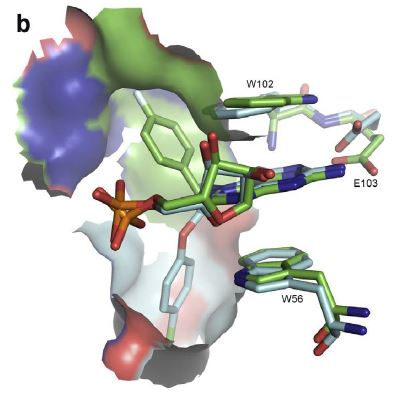
N7-substituents other than methyl, such as 4-fluorobenzyl [24] (green) or (4-chlorophenoxy)ethyl [20] (cyan), also make hydrophobic contacts with two concave lipophilic pockets
(surface representations) behind the W56eW102 stack. Figure constructed from PDB entries 2V8W, 2V8Y, 4DT6, and 4TPW. 3D-Structure illustrations in this and subsequent
Figures were prepared using MacPyMOL (The PyMOL Molecular Graphics System, Version 1.2, Schr€odinger, LLC). (For interpretation of the references to colour in this figure legend,
the reader is referred to the web version of this article.)

![Fig. 1. (a) mRNA cap recognition (represented by m7GTP shown as yellow CPK sticks) and binding of eIF4G or 4E-BPs (represented by a peptide derived from 4E-BP1 shown as a cyan cartoon) occurs on opposite faces of eIF4E (green cartoon). Apart from direct cap-binding antagonists, allosteric inhibitors (binding pose of 4-EGI1 [22] shown as magenta sticks) and inhibitors derived from eIF4G and 4E-BPs [23] are being developed. Whereas m7GTP-binding is dominated by polar interactions between the cationic N-methylpurine system and eIF4E residues W56, W102, and E103 (cationep interaction and H-bonds), as well as the phosphate groups with residues R157, K159, and K162,](https://blogs.nottingham.ac.uk/pharmacy/files/2017/05/Journal-sq-150x150.jpg)